Computational strategies for amyloidogenic proteins interacting with gold nanoparticles
Giorgia Brancolini1, Stefano Corni1
1CNR-NANO S3, Institute of Nanoscience, via Campi 213/A, 41100 Modena, Italy
E-mail: This email address is being protected from spambots. You need JavaScript enabled to view it.
Nanoparticles (NPs) are recognized to exhibit distinct physical and chemical properties compared with the same materials in the bulk form. [1] NPs have been repeatedly reported to interact with proteins, and this interaction can be exploited to affect processes undergone by proteins, such as fibrillogenesis. Fibrillation is common to many proteins, and in living organisms, it causes tissue-specific or systemic amyloid diseases.
We present recent computational modeling advances which were pursued in the quest for a theoretical framework elucidating the association mechanisms and the ability to design and control the recognition capability of β2-microglobulin towards (i) 5 nm hydrophilic citrate-capped gold nanoparticles (Fig.1) and (ii) 2.5 nm gold nanoparticles functionalized with hydrophobic organic ligands. Recent simulations at multiple levels (enhanced sampling molecular dynamics, Brownian dynamics, and Poisson_Boltzmann electrostatics) and NMR measurements have explained the origin of the observed protein perturbations in the presence of citrate-capped gold NPs. Experiments have shown that the interaction is weak in the physiological-like, conditions and it is not able to induce protein fibrillation. [2] On the contrary, binding of the protein to hydrophobic ligands can be stronger and able to block the active sites of domain from binding to another protein, thus inhibiting the fibrillation activity.[3]
The interaction of the gold-nanoparticles with two amyloidogenic variants of β2-microglobulin is further discussed, namely (a) the truncated ΔN6 and (b) the mutated D76N, and a fibrillization pathway for them is proposed. The results offer possible strategies for controlling the desired effect of NPs on the conformational changes of amyloidogenic proteins, which have crucial roles in the fibrillation process.[4]
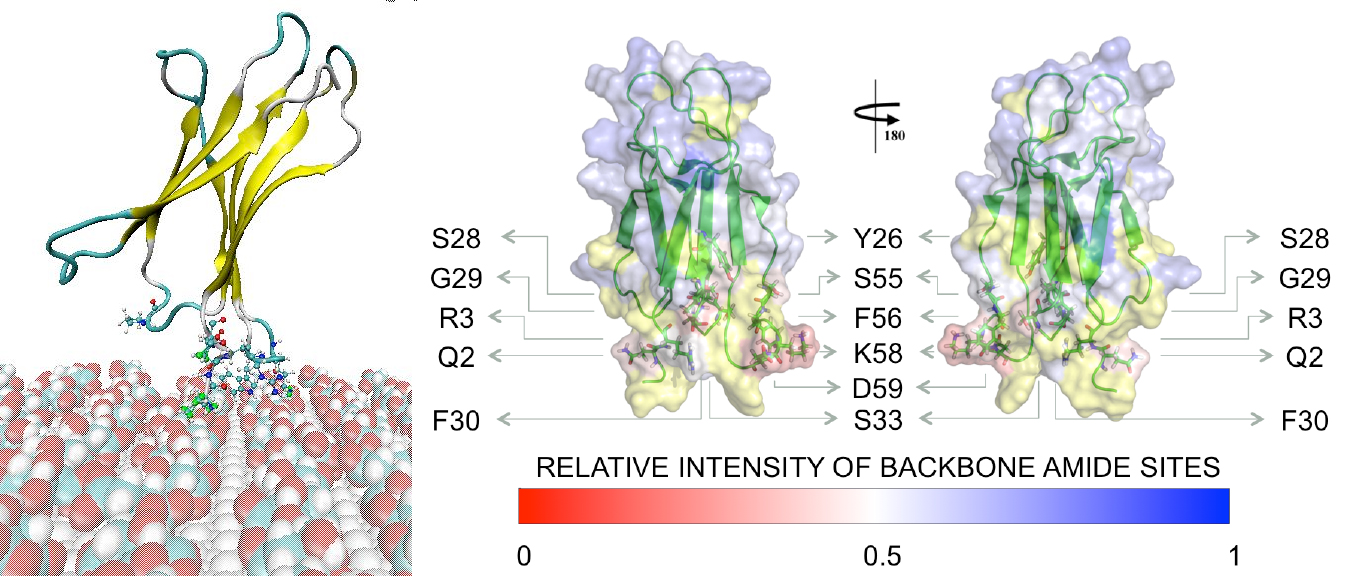
Fig. 1 Monomeric protein interacting with citrate-capped gold nanoparticle: (left) 20 ns of T-REMD simulations (right) NMR protein signal intensities
------------
[1] Kumar et al., 2013. Manual on Critical Issues in Nanotechnology R&D Management: An Asia-Pacific Perspective. APCTT-ESCAP
[2] Brancolini, G. et al. Nanoscale, 2014, 6, 7903-7911
[3] (i) Brancolini, G. et al. ACS NANO, 2015, 9, 2600-2613 (ii) Brancolini, G. et al. ACS Nano, 2012, 6, 9863-9878
[4] (i) Mangione, P. P. et al., J. Biol. Chem. 2013, 288, 30917-30930 (ii) G. Esposito et al. Subcell Biochem. 2012, 65, 165-83.



